Blank Titration Definition Chemistry . it is used in quantitative analytical chemistry to determine an unknown concentration of an identified. The only difference from the. Learn the titration graph and. What are titrant and analyte. a blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. What is the equivalence point of a titration. the type of reaction provides us with a simple way to divide titrimetry into four categories:
from www.chemistryscl.com
What are titrant and analyte. The only difference from the. a blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. Learn the titration graph and. the type of reaction provides us with a simple way to divide titrimetry into four categories: What is the equivalence point of a titration. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. it is used in quantitative analytical chemistry to determine an unknown concentration of an identified. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of.
Titrimetry, Titration Classifications, Standard solutions, Equivalence
Blank Titration Definition Chemistry the type of reaction provides us with a simple way to divide titrimetry into four categories: titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. What is the equivalence point of a titration. The only difference from the. What are titrant and analyte. it is used in quantitative analytical chemistry to determine an unknown concentration of an identified. the type of reaction provides us with a simple way to divide titrimetry into four categories: Learn the titration graph and. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant.
From www.savemyexams.com
Titrations (5.6.3) AQA A Level Chemistry Revision Notes 2017 Save Blank Titration Definition Chemistry the type of reaction provides us with a simple way to divide titrimetry into four categories: a blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution. Blank Titration Definition Chemistry.
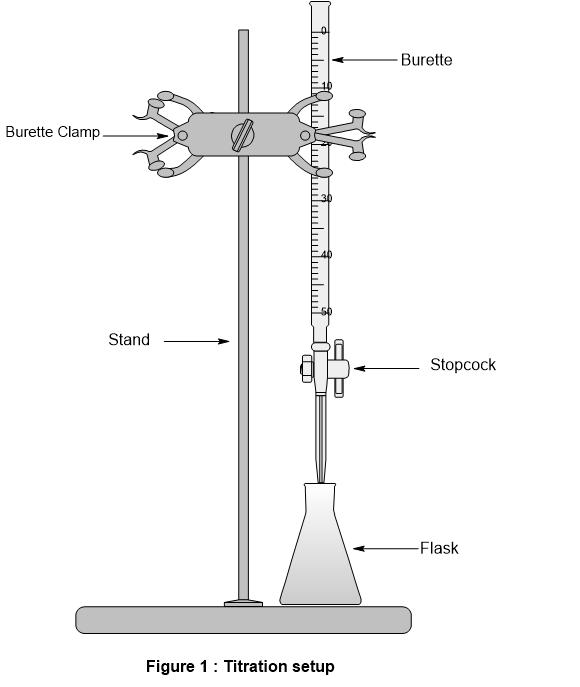
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Blank Titration Definition Chemistry What is the equivalence point of a titration. Learn the titration graph and. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. the type of reaction provides us with a simple way to divide titrimetry into four categories: What are titrant and analyte.. Blank Titration Definition Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Blank Titration Definition Chemistry titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Learn the titration graph and. a blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. the type of reaction provides us with a. Blank Titration Definition Chemistry.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Blank Titration Definition Chemistry a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Learn the titration graph and. What are titrant and analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Web. Blank Titration Definition Chemistry.
From shelbyscrosbyo.blob.core.windows.net
Titration Method Definition at shelbyscrosbyo blog Blank Titration Definition Chemistry a blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. The only difference from the. it is used in quantitative analytical chemistry to determine an unknown concentration of an identified. Learn the titration graph and. titration is the slow addition of one solution of a known. Blank Titration Definition Chemistry.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Blank Titration Definition Chemistry What are titrant and analyte. What is the equivalence point of a titration. The only difference from the. Learn the titration graph and. a blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. titration is the slow addition of one solution of a known concentration (called a. Blank Titration Definition Chemistry.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Blank Titration Definition Chemistry The only difference from the. What is the equivalence point of a titration. the type of reaction provides us with a simple way to divide titrimetry into four categories: Learn the titration graph and. a blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. it is. Blank Titration Definition Chemistry.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Blank Titration Definition Chemistry a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. it is used in quantitative analytical chemistry to determine an unknown concentration of an identified. Learn the titration graph and. What are titrant and analyte. the type of reaction provides us with a. Blank Titration Definition Chemistry.
From facts.net
8 Captivating Facts About AcidBase Titration Blank Titration Definition Chemistry Learn the titration graph and. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. The only difference from the. Web. Blank Titration Definition Chemistry.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Blank Titration Definition Chemistry What are titrant and analyte. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. it is used in quantitative. Blank Titration Definition Chemistry.
From labthink.netlify.app
What is titration and how does it work Blank Titration Definition Chemistry a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Learn the titration graph and. a blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. What are titrant and analyte. it is. Blank Titration Definition Chemistry.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Blank Titration Definition Chemistry a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a blank titration is carried out by titrating a fixed. Blank Titration Definition Chemistry.
From hubpages.com
Different Methods of Measuring Drug Potency, Concentration, Efficacy Blank Titration Definition Chemistry a blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. What is the equivalence point of a titration. The only difference from the. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant.. Blank Titration Definition Chemistry.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Blank Titration Definition Chemistry it is used in quantitative analytical chemistry to determine an unknown concentration of an identified. the type of reaction provides us with a simple way to divide titrimetry into four categories: What is the equivalence point of a titration. What are titrant and analyte. The only difference from the. a titration is a volumetric technique in which. Blank Titration Definition Chemistry.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Blank Titration Definition Chemistry a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. What are titrant and analyte. the type of reaction provides us with a simple way to divide titrimetry into four categories: it is used in quantitative analytical chemistry to determine an unknown concentration. Blank Titration Definition Chemistry.
From mungfali.com
Acid Base Titration Method Blank Titration Definition Chemistry a blank titration is carried out by titrating a fixed and known concentration of titrant into a solvent with zero analyte. What are titrant and analyte. the type of reaction provides us with a simple way to divide titrimetry into four categories: it is used in quantitative analytical chemistry to determine an unknown concentration of an identified.. Blank Titration Definition Chemistry.
From www.researchgate.net
Blank titrations with ATPγS in the absence of any protein (A Blank Titration Definition Chemistry a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. the type of reaction provides us with a simple way to divide titrimetry into four categories: What is the equivalence point of a titration. a blank titration is carried out by titrating a. Blank Titration Definition Chemistry.
From www.osmosis.org
Introduction to titrations Vídeo & Anatomía Osmosis Blank Titration Definition Chemistry titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. the type of reaction provides us with a simple way to divide titrimetry into four categories: it is used in quantitative analytical chemistry to determine an unknown concentration of an identified. What are titrant. Blank Titration Definition Chemistry.